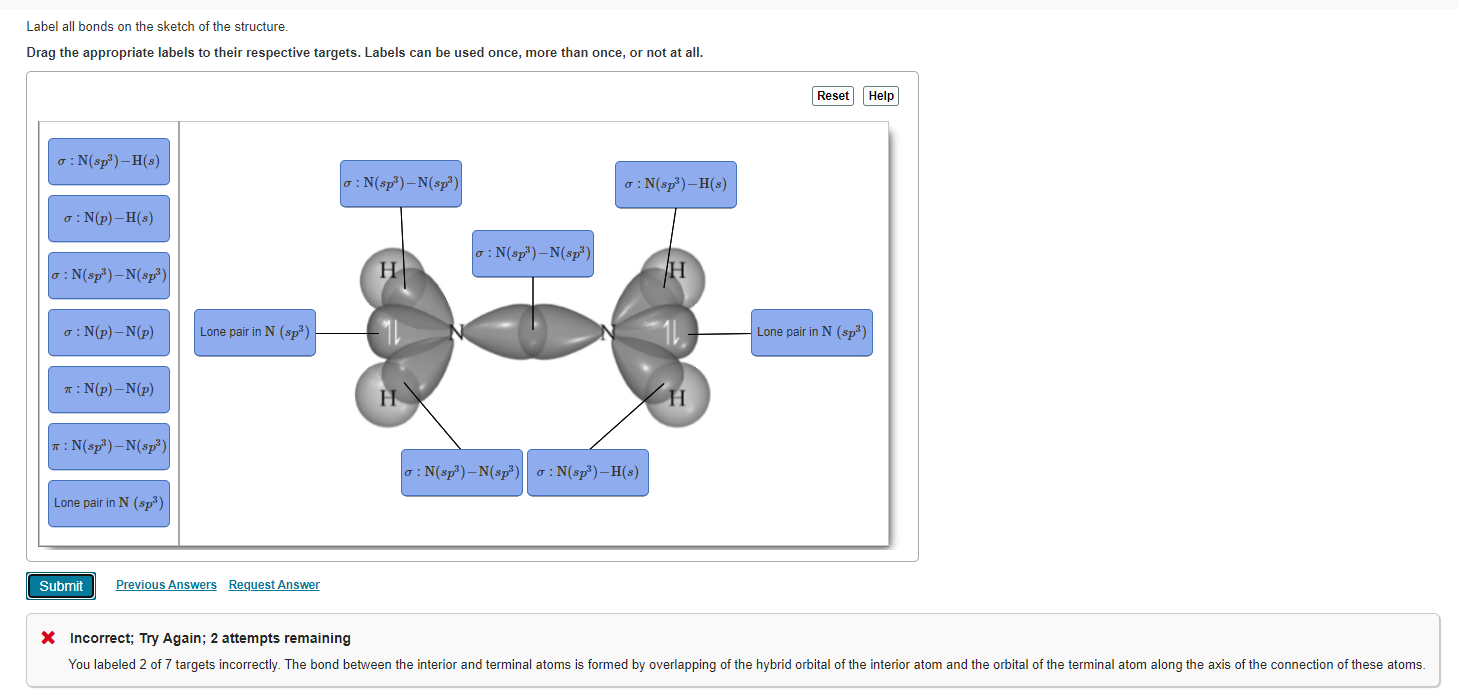
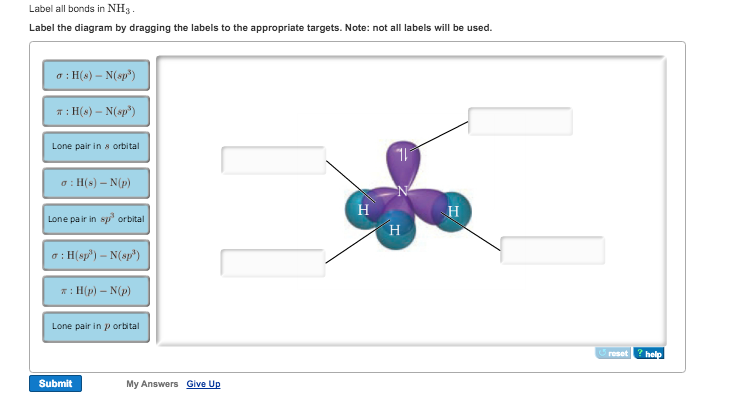
39 label all bonds on the sketch of the structure.
Chapter 11, Chemical Bonding II: Molecular Shapes, Valence Bond Video ... Write a hybridization and bonding scheme for each molecule that contains more than one interior atom. Indicate the hybridization about each interior atom. Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 11.6 and 11.7 a. $\mathrm{N}_{2} \mathrm{H}_{2}$ (skeletal structure $\mathrm ... SOLVED:Write a hybridization and bonding scheme for each ... - Numerade Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. ... and label all bonds using the notation shown in Examples 6.1 and 6.2. a. CCl4 b. NH3 c. OF2 d. CO2. Answer (a) See solution (b) See solution (c) See solution ... So if we drew our traditional Zohra will a structure if ...
NH3 Lewis Structure, Molecular Geometry, Hybridization, Bond Angle & Shape NH3 Bond angles. There are three single bonds and one lone pair of electrons in NH3 molecule. It has a molecular geometry of trigonal pyramidal which also looks like a distorted tetrahedral structure. The shape is distorted because of the lone pairs of electrons. This pair exerts repulsive forces on the bonding pairs of electrons.

Label all bonds on the sketch of the structure.
Chapter 10: Chemical Bonding II: Molecular Shapes, Valence Bond Theory ... Tap card to see definition 👆. Combines the Lewis Model with the idea that valence electron groups repel one another (Covalent Bonds) to predict the general shape of a molecule from its Lewis Structure. Repulsions between electron groups on the interior atoms of a molecule determine the geometry of the molecule, and the preferred geometry is ... SOLVED:Write a hybridization and bonding scheme for each molecule or ... Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. COCl2 (carbon is the central atom) b. BrF5 c. XeF2 d. I3 Answer a) The interior C atom has three electron groups. So the electron geometry is trigonal planar and the hybridization is s p 2 . Use valence bond theory to write the hybridization and ... - Socratic Step 1. Draw the Lewis structure (a) Start with a skeleton structure. The two #"C"# atoms (least electronegative) will be the central atoms, with the #"N"# attached to one of the carbons. (b) Attach the hydrogen atoms. The question gives you a clue where they go. The formula #"NCCH"_3# tells you that the three #"H"# atoms are attached to the terminal carbon atom.
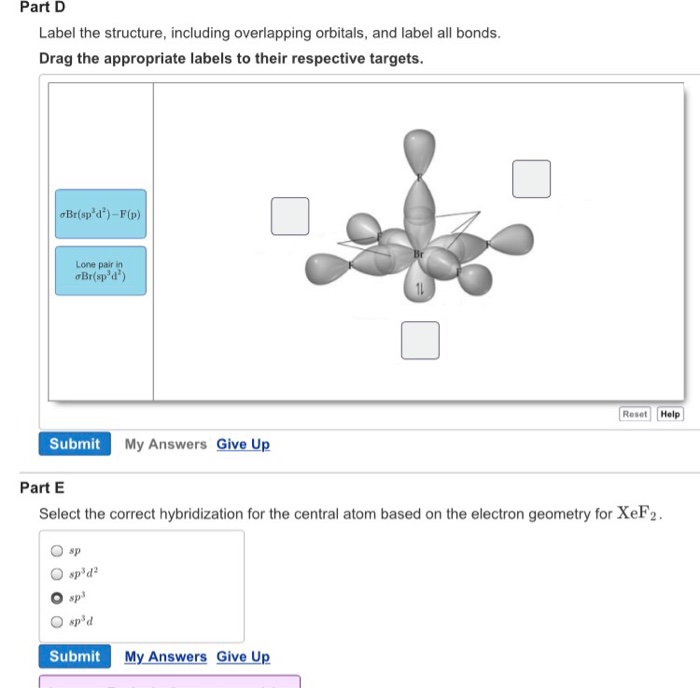
Label all bonds on the sketch of the structure.. Chapter 6, Chemical Bonding II Video Solutions, Chemistry - Numerade Write a hybridization and bonding scheme for each molecule that contains more than one interior atom. Indicate the hybridization about each interior atom. Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. C2H2 (skeletal structure HCCH) b. Chemistry: Semester 2, Unit 1 Practice Problems - Quizlet Sketch the structure, including overlapping orbitals, and label all bonds including the notation shown in examples 10.6 and 10.7 a. COCl2 (carbon is central atom b. BrF5 c. XeF2 d. I3- ... 65: Write a hybridization and bonding scheme for each molecule that contains more than one interior atom. Indicate the hybridization about each interior atom. Label All Bonds In Bf3 - Structure And Bonding Of New Boron And Carbon ... Label all bonds in ch2br2 label the diagram by dragging the labels to the appropriate targets. All eyes on the bond market as global yields continue to fall. Note that not all labels will be used. Solution for in the sketch of the structure of bf3 label all bonds. Drag the appropriate labels to their respective targets. Post Lecture Lewis Structures, Problem Set #6 (Ch 5), Problem ... - Quizlet Draw an appropriate Lewis structure for IF5. Identify the geometry of IF5 using VSEPR theory. Specify whether the molecule IF5 is polar or nonpolar and explain why. Identify the hybridization of all interior atoms for the molecule IF5, according to valence bond theory, in the diagram showing orbital overlap below.
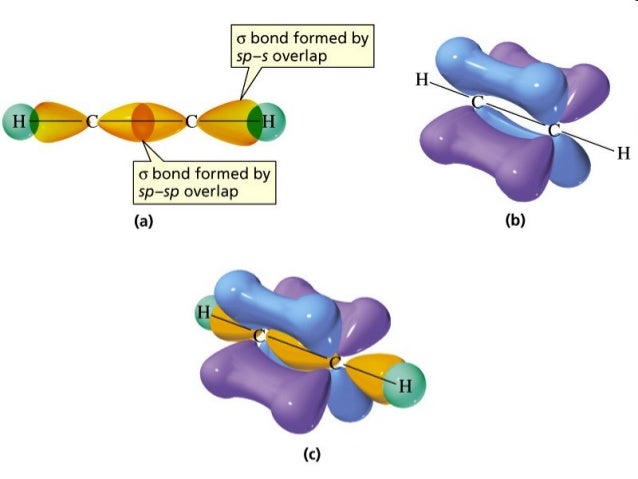
SOLVED:Write a hybridization and bonding scheme for each ... - Numerade Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. SO3 2- b. PF6- c. BrF3 d. HCN Answer a) The S atom has four electron groups. So the electron geometry is tetrahedral and the hybridization is s p 3 . b) The P atom has six electron groups with no lone pairs. SOLVED:Write a hybridization and bonding scheme for each ... - Numerade Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. C2H2 (skeletal structure HCCH) b. C2H4 (skeletal structure H2CCH2) c. C2H6 (skeletal structure H3CCH3) Answer. a) Both the C atoms have two electron groups. So the electron geometry is linear and the (Solved) : Label Bonds Ch2br2 Label Bonds So2 Label Bonds Nf3 Label ... Label all bonds in NF3. Label all bonds in BF3. Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. o C(sp) H(s) o C(sp') Br(s) C(p) H(p) C(p) Br(p) C(sp) H(p) o C(sps) Br (p) C(sps) Br (p) reset help Show transcribed image text Expert Answer SOLVED:Write a hybridization and bonding scheme for each ... - Numerade Problem 34 Hard Difficulty. Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2.
CHEM: Chapter 10 Flashcards | Quizlet Determine the geometry about each interior atom in each molecule and sketch the molecule. (Skeletal structure is indicated in parentheses.) a. CH3NH2 (H3CNH2) b. CH3CO2CH3 (H3CCOOCH3 One O atom attached to 2nd C atom; the other O atom is bonded to the 2nd and 3rd C atom) c. NH2CO2H (H2NCOOH both O atoms attached to C) Molecular Shape and Polarity OneClass: Label all bonds in CH2Br2? Label all bonds in CH 2 Br 2? Answer +20. Watch. 1. answer. 2. watching. 628. views. For unlimited access to Homework Help, a Homework+ subscription is required. ... Chemistry: Structure and Properties. 2nd Edition, Tro. ISBN: 9780134293936. Related questions. Which compound contains both ionic and covalent bonds? A. KI. B. CaCl 2. C. CH 2 Br 2. Use valence bond theory to write the hybridization and ... - Socratic Step 1. Draw the Lewis structure (a) Start with a skeleton structure. The two #"C"# atoms (least electronegative) will be the central atoms, with the #"N"# attached to one of the carbons. (b) Attach the hydrogen atoms. The question gives you a clue where they go. The formula #"NCCH"_3# tells you that the three #"H"# atoms are attached to the terminal carbon atom. SOLVED:Write a hybridization and bonding scheme for each molecule or ... Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. COCl2 (carbon is the central atom) b. BrF5 c. XeF2 d. I3 Answer a) The interior C atom has three electron groups. So the electron geometry is trigonal planar and the hybridization is s p 2 .
Chapter 10: Chemical Bonding II: Molecular Shapes, Valence Bond Theory ... Tap card to see definition 👆. Combines the Lewis Model with the idea that valence electron groups repel one another (Covalent Bonds) to predict the general shape of a molecule from its Lewis Structure. Repulsions between electron groups on the interior atoms of a molecule determine the geometry of the molecule, and the preferred geometry is ...


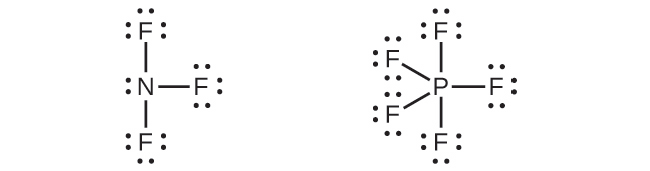
Post a Comment for "39 label all bonds on the sketch of the structure."