45 label all bonds in ch2br2
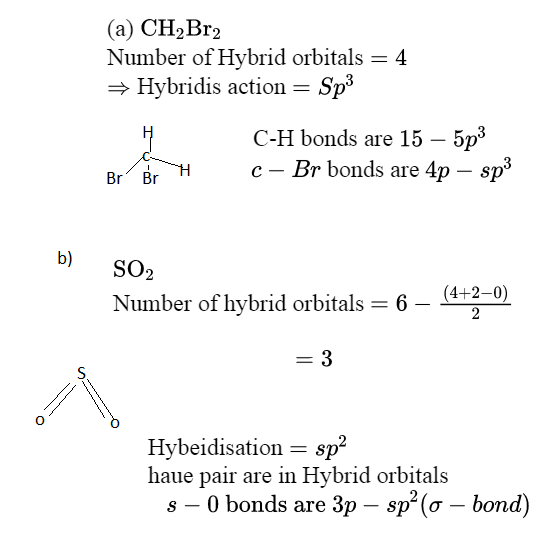
CH2Br2 Lewis Structure, Geometry, Hybridization, and Polarity For CH2Br2, there are 4 electron pairs. 6. Divide the total electron pairs as bonding and non-bonding. The bonding electron pair is equal to the number of side atoms. For CH2Br2, there are four side atoms. Thus, there are four bonding pairs of electrons and zero nonbonding pairs of electrons. Answered: Write a hybridization and bonding… | bartleby CH2Br2 b. SO2 c. NF3 d. BF3 Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. a. CH2Br2 b. SO2 c. NF3 d. BF3 Question Write a hybridization and bonding scheme for each molecule.
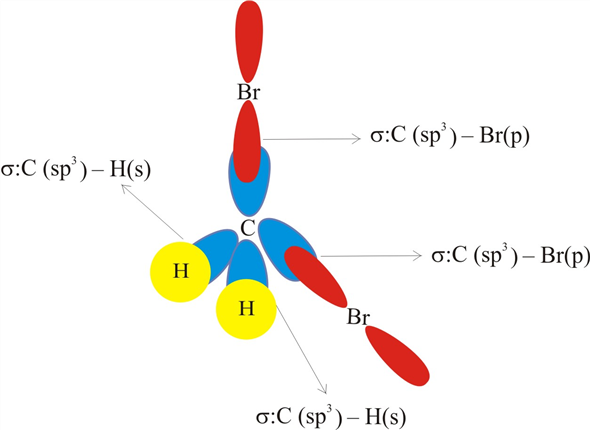
Answered: In the sketch of the structure of… | bartleby In the sketch of the structure of CH2B12 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. : C (sp³) - Br (p) o : C (sp*) - H (s) H : C (sp³) - Br (s) T: C (p) - Br (p) H o : C (sp³) - H (p) Br Br T: C (p) - H (p) o : C (sp³) - Br (p) *: C (sp³) - H (p) BUY

Label all bonds in ch2br2
CHEM: Chapter 10 Flashcards | Quizlet CH3NH2 (H3CNH2) b. CH3CO2CH3 (H3CCOOCH3 One O atom attached to 2nd C atom; the other O atom is bonded to the 2nd and 3rd C atom) c. NH2CO2H (H2NCOOH both O atoms attached to C) Molecular Shape and Polarity 52. Determine whether each molecule is polar or nonpolar. a. SiCl4 b. CF2Cl2 c. SeF6 d. IF5 a) non-polar Molecular orbital diagram for BF3 - Chemistry Stack Exchange 2p (along the bond axis) for F has an irreducible representation of E' and A' 2p (z) for F Has an irreducible representation of E'' and A''2 2p (the remaining one) has an irreducible representation of E' and A'2 . From this I built A1 bonding and antibonding orbitals, A'2 bonding and antibonding orbitals, 1 A'2 and 2 E''2 non bonding orbitals. Finding the hybridization of atoms in organic molecules (worked ... When I get to the triple bond, I know one of those is a sigma bond, and two of those are pi bonds. So, two of those are pi bonds, here. And then, finally, I have one more bond; it's a single-bond, so I know that it is a sigma bond here, and if you count up all of those sigma bonds, you should get 10, so let's do that really quickly.
Label all bonds in ch2br2. Label All Bonds In Bf3 - Which Triel Bond Is Stronger Trhx H2y Versus ... Label all bonds in ch2br2 label the diagram by dragging the labels to the appropriate targets. During the wednesday morning trading session, traders were closely watching the treasuries market. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in examples 6.1 and 6.2. ... Write a hybridization and bonding scheme for each molecule ... Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. CH2Br2 b. SO2 c. NF3 d. BF3 ... Label all bonds in SO2. - Transtutors Nov 18, 2022 ... Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. o ... Write a hybridization and bonding scheme for each molecule ... Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. \begin{equation}\text { a. }\ ...
Solved In the sketch of the structure of CH2 Br2 label all - Chegg Expert Answer 80% (20 ratings) Transcribed image text: In the sketch of the structure of CH2 Br2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. CH2Br2 Molecular Geometry - Science Education and Tutorials The formula of CH2Br2 molecular hybridization is as follows: No. Hyb of CH2Br2= N.A (C-H and C-Br bonds) + L.P (C) No. Hy of CH2Br2= the number of hybridizations of CH2Br2 Number of C-H and C-Br bonds = N.A (C-Br and C-H bonds) Lone pair on the central carbon atom = L.P (C) Calculation for hybridization number for CH2Br2 molecule Solved Label all bonds in CH2Br2. Label all bonds in | Chegg.com Transcribed image text: Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. o C (sp) H (s) o C (sp') Br (s) C (p) H (p) C (p) Br (p) C (sp) H (p) o C (sps) Br (p) C (sps) Br (p) reset help Previous question Next question Chapter 6, Chemical Bonding II Video Solutions, Chemistry: Structure ... Chemical Bonding II Chemistry: Structure and Properties Nivaldo Tro Chapter 6 Chemical Bonding II - all with Video Answers Educators AM PH + 2 more educators Chapter Questions 00:44 Problem 1 Why do we use other bonding theories in addition to the Lewis model? Joshua Speer Numerade Educator 00:30 Problem 2
How many bonds are in CH2Br2? - Answers What is the name of CH2Br2? dibromomethane Does CH2Br2 form hydrogen bonds? No, hydrogen bonding only occurs in compounds where hydrogen (H) is bonded to nitrogen (N), oxygen (O) or fluorine... [Solved] Label all bonds in SO2. Label all bonds i | SolutionInn Transcribed Image Text: Label all bonds in CH₂ Br2. Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. a: C (sp³) - H (s) σ: C (sp³) - Br (s) ㅠ: T: C (p) - H (p) TT: C (p) - Br (p) T: C (sp³) - H (p) σ: C (sp³) - H (p) σ: C (sp³) - Br (p) T: C (sp³) - Br (p) Br H H Br reset ? help (Solved) - Part B Label all bonds in CH2Br2 Label the diagram by ... Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note. The molecule is CH2Br2. Br H C Br H dibromomethane The molecule is a derivative of methane. The central atom carbon is bonded to... Posted one year ago Q: p-Methylbenzonitrile Complete the scheme by dragging the labels to the appropriate targets. Quickly Determine The sp3, sp2 and sp Hybridization - Chemistry Steps The best example is the alkanes. All the carbon atoms in an alkane are sp 3 hybridized with tetrahedral geometry. The carbons in alkenes and other atoms with a double bond are often sp 2 hybridized and have trigonal planar geometry. The triple bond, on the other hand, is characteristic for alkynes where the carbon atoms are sp-hybridized. Some ...
CH2Br2 Lewis Structure - Learnool CH 2 Br 2 lewis structure. CH 2 Br 2 (dibromomethane) has one carbon atom, two hydrogen atoms, and two bromine atoms. In the lewis structure of CH 2 Br 2, there are four single bonds around the carbon atom, with two hydrogen atoms and two bromine atoms attached to it, and on each bromine atom, there are three lone pairs. Steps.
Label All Bonds In Bf3 / Bf3 Molecular Geometry Science Education And ... Label all bonds in ch2br2 label the diagram by dragging the labels to the appropriate targets. There exists 3 regions of electron density, all of which are single covalent bond between the boron atom and the fluorine . Note that not all labels will be used. During the wednesday morning trading session, traders were closely watching the ...
Label all bonds in CH2Br2. Label all bonds in SO2. Label all bonds in ... Label all bonds in CH2Br2. Label all bonds in SO2. Label all bonds in NF3. Label… The following solution is suggested to handle the subject "Label all bonds in CH2Br2. Label all bonds in SO2. Label all bonds in NF3. Label…". Let's keep an eye on the content below! Question "Label all bonds in CH2Br2. Label all bonds in SO2. Label ...
soc - VSEPR and MO Diagrams Workshop Activity - Studocu In the sketch of the structure of label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. ANSWER: sp 2 sp 3 s p 3 d C C H 2 B r 2 sp sp 2 sp 3 s p 3 d CH 2 Br 2 Part C Identify the hybridization of the atom in. ANSWER: Correct
OneClass: Label all bonds in CH2Br2? Label all bonds in CH 2 Br 2? + 20 Watch For unlimited access to Homework Help, a Homework+ subscription is required. Kottherva Sreevidya Lv10 5 Jan 2021 Unlock all answers Get 1 free homework help answer. Unlock Already have an account? Log in Like Ask a question Chemistry: The Central Science Weekly leaderboard Home Homework Help 3,800,000
Dibromomethane | CH2Br2 - PubChem In laboratory studies, animals experienced CNS depression at 2400-2800 ppm and liver and kidney damage after repeated exposures to 1000 ppm. [CHEMINFO] Dichloromethane seldom causes hepatotoxicity unless exposure is very heavy or agent ingested. [Zimmerman, p. 333] If left on clothes, may cause reddening of skin; [CHRIS] May have effects on nervous system and blood, causing impaired functions ...
Label all bonds in SO_2. Label the diagram by dragging the labels to ... Label all bonds in SO_2. Label the diagram by dragging the labels to the appropriate targets. Valence Bond Theory. In discussing covalent bonding, the Valence Bond Theory describes bonding within a molecule as overlapping atomic orbitals. In this theory, atomic orbitals of individual atoms in a molecule hybridize to form new orbitals during ...
SO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram The molecular geometry of SO2 is bent, with a bond angle of 120°. We can easily find out the molecular geometry of any compound using the given chart. Here, A = central atom, X = surrounding atoms and E = the lone pairs. SO2 is an AX2E type molecule, with 2 surrounding atoms i.e oxygen, and 1 lone pair of sulfur.
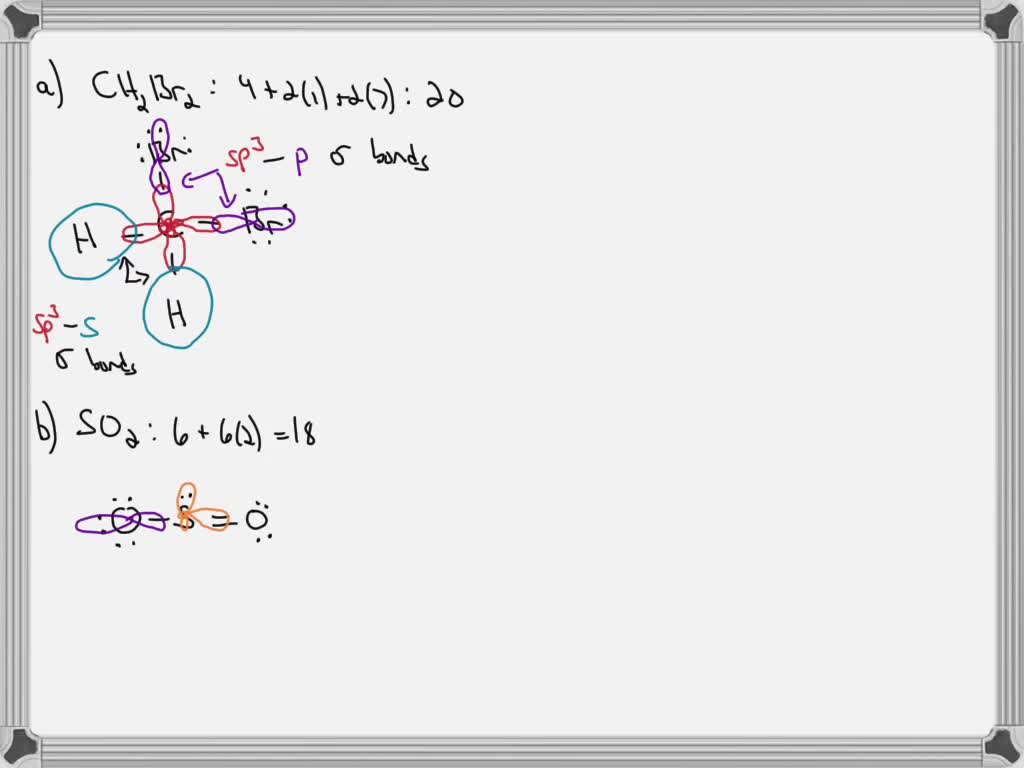
Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2., a. CH2Br2, b. ...
Write a hybridization & bonding scheme for each molecule. Sketch each ... Sketch each molecule, including overlapping orbitals and label all bonds. a. CH2Br2 b. SO2 a. C H 2 B r 2 b. S O 2 Orbital Hybridization and Bonding: Orbital hybridization is the process of...
CH2Br2 Lewis Structure & Characteristics (17 Complete Facts) Let us study the facts about CH 2 Br 2 in more detail. Dibromomethane (CH2Br2) is a clear liquid that has a pleasant smell. With a melting point of -52.7 °C, or 62.8 °F, and a molecular weight of 173.8 g/mol, the substance has a boiling point between 96 °C and 98 °C. A density of 2.477 g/mL makes CH2Br2 denser than water.
Dibromomethane | CH2Br2 - PubChem Dibromomethane | CH2Br2 | CID 3024 - structure, chemical names, ... Hydrogen Bond Donor Count, 0, Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07).
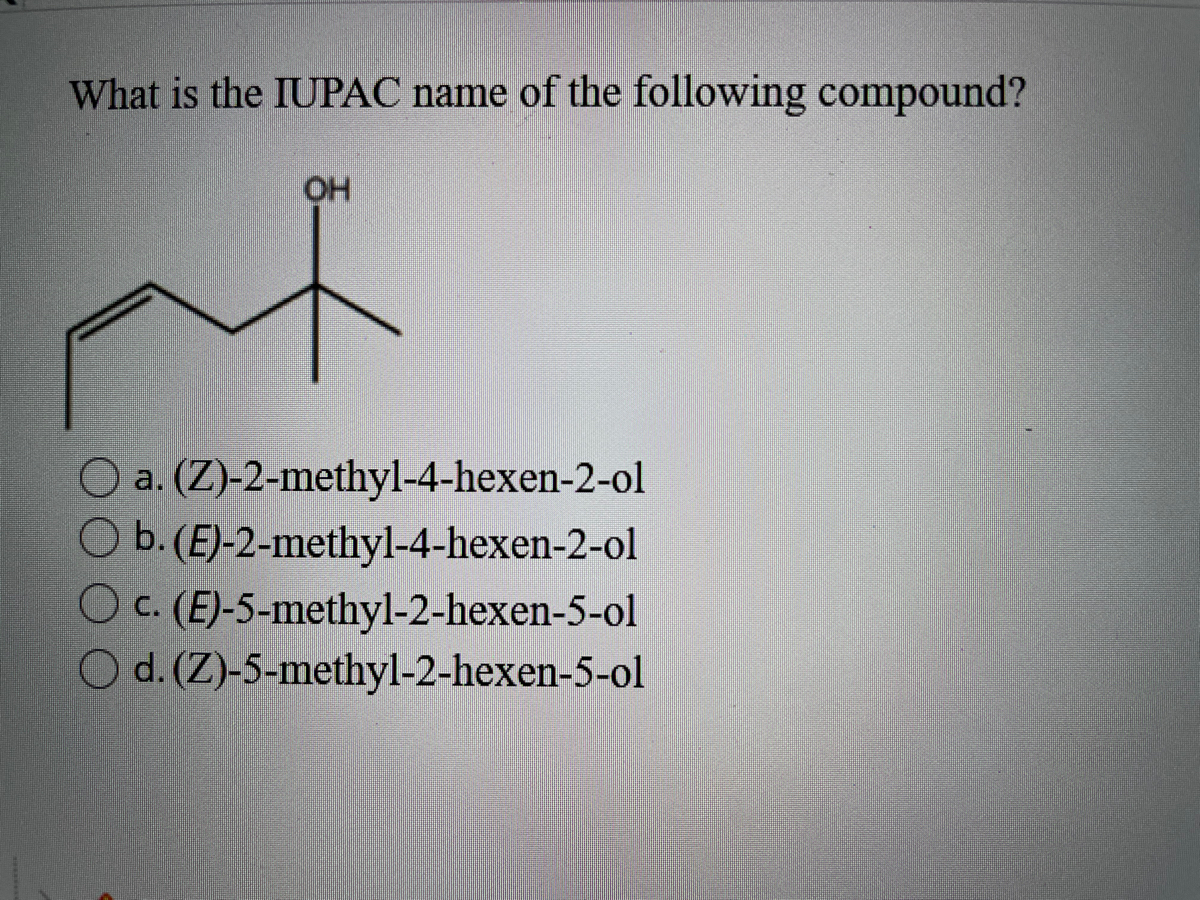
Use valence bond theory to write the hybridization and ... - Socratic Warning! Long Answer. Here's what I get. > Step 1. Draw the Lewis structure (a) Start with a skeleton structure. The two "C" atoms (least electronegative) will be the central atoms, with the "N" attached to one of the carbons. (b) Attach the hydrogen atoms. The question gives you a clue where they go. The formula "NCCH"_3 tells you that the three "H" atoms are attached to the terminal carbon atom.
(Solved) - Label all bonds in CH2Br2 Label the diagram by dragging the ... Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. o C (sp) H (s) o... Posted 4 months ago Q: Transcribed image text : Label all bonds in BF3. Label the diagram by dragging the labels to the appropriate targets.
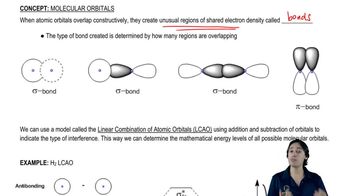
Chapter 10: Chemical Bonding II: Molecular Shapes, Valence Bond Theory ... Single Bonds, Double Bonds, Triple Bonds, Lone Pairs, and Free Radicals all count as one Electron Group Electron Geometry Doesn't distinguish between Nonbonding and Bonding Electron Groups (Ideal) Molecular Geometry The actual geometrical arrangement of electrons, based on the type of electron groups in the molecule
Finding the hybridization of atoms in organic molecules (worked ... When I get to the triple bond, I know one of those is a sigma bond, and two of those are pi bonds. So, two of those are pi bonds, here. And then, finally, I have one more bond; it's a single-bond, so I know that it is a sigma bond here, and if you count up all of those sigma bonds, you should get 10, so let's do that really quickly.
Molecular orbital diagram for BF3 - Chemistry Stack Exchange 2p (along the bond axis) for F has an irreducible representation of E' and A' 2p (z) for F Has an irreducible representation of E'' and A''2 2p (the remaining one) has an irreducible representation of E' and A'2 . From this I built A1 bonding and antibonding orbitals, A'2 bonding and antibonding orbitals, 1 A'2 and 2 E''2 non bonding orbitals.
CHEM: Chapter 10 Flashcards | Quizlet CH3NH2 (H3CNH2) b. CH3CO2CH3 (H3CCOOCH3 One O atom attached to 2nd C atom; the other O atom is bonded to the 2nd and 3rd C atom) c. NH2CO2H (H2NCOOH both O atoms attached to C) Molecular Shape and Polarity 52. Determine whether each molecule is polar or nonpolar. a. SiCl4 b. CF2Cl2 c. SeF6 d. IF5 a) non-polar



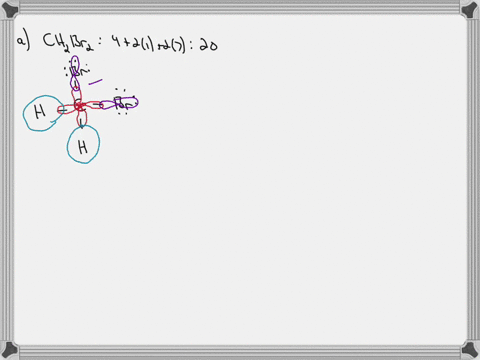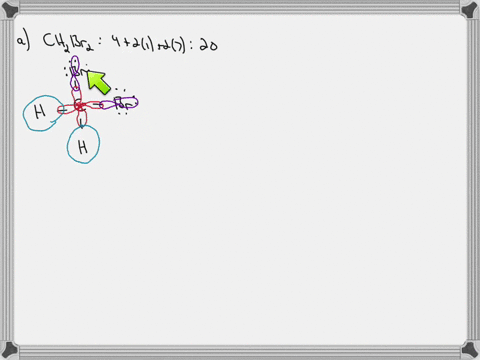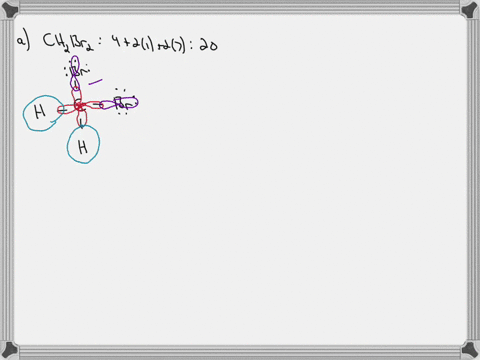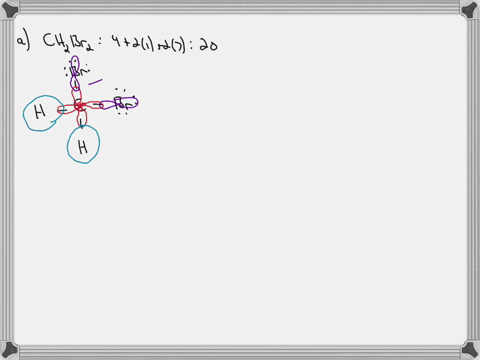








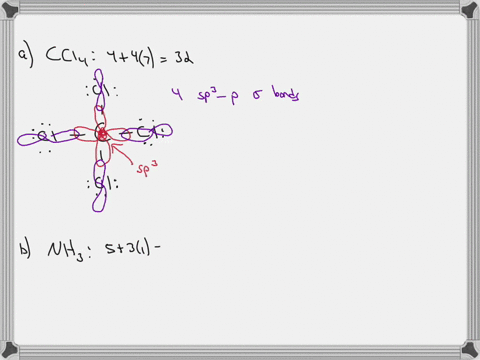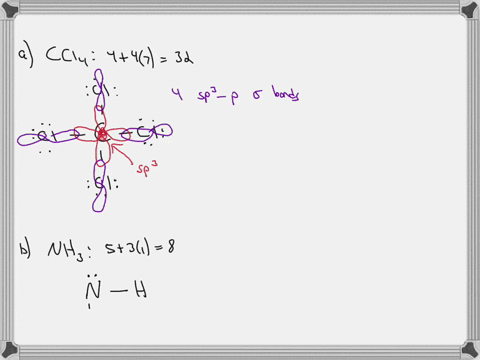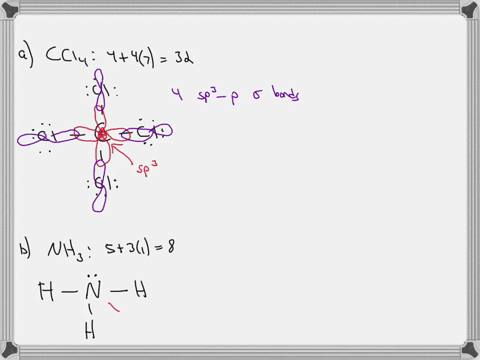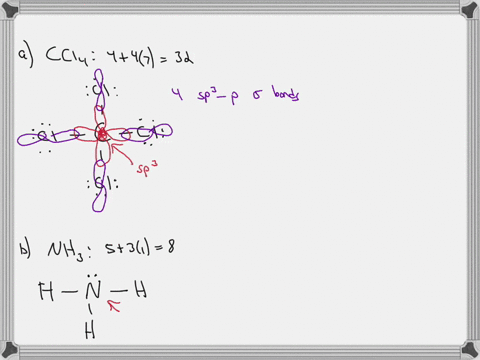











Post a Comment for "45 label all bonds in ch2br2"